Summary: Abbott’s Triclip™ is one-of-its-kind transcatheter valve repair clip device designed for patients with multiple co-morbidities. The non-surgical treatment alternative prevents the probability of high-risk open-heart surgery and improves quality of life for people with severe and symptomatic tricuspid regurgitation.
Cardiovascular diseases are one of the leading causes of death worldwide, but most of them could be prevented with proper medical interventions. However, the introduction of smart applications, remote monitoring, and minimally invasive approaches have been successful in targeting the gaps in vascular health care through technology and saving millions of lives. Many new treatment options are being developed to eliminate the need of open-heart surgery as it carries a high degree of risk. The advent of transcatheter tricuspid valve intervention (TTVI) devices as an alternative treatment option for tricuspid regurgitation (TR) or the leaky tricuspid valve have revolutionized the field of structural cardiology. Designed to reduce TR severity by valve leaflet plication, the percutaneous coronary devices have gained a lot of recognition due to their ability to treat severe TR in a minimally invasive way without posing the risk of severe complications. Currently, 80% of mitral regurgitation treatments are being performed with the MitraClip/TriClip™ system worldwide to address the specific features of the transcupid valve anatomy.
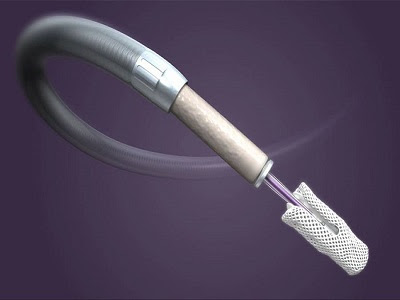
Abbott's TriClip™ is the first-of-its-kind transcatheter tricuspid valve repair clip device to receive CE mark clearance as a non-surgical treatment option for people with TR. The minimally invasive clip-based device is an iteration of the company's highly popular and successful MitraClip used for transcatheter mitral valve. The next-generation clip-based therapy, also known as TriClip™ G4 consists of a leaflet grasping feature and new clip sizes to accommodate and tailor repair of the unique anatomy of each patient. Leaving TR unaddressed for a long period can lead to atrial fibrillation, heart failure, or even death so Abbott's TriClip™ serves as a suitable option for patients with multiple co-morbidities. Thus, the device prevents the high-risk patients undergo the open-heart surgery, which could present a number of complications. The new-generation clip therapy can enhance the cardiologists' ability to repair tricuspid valves safely and effectively, thus dramatically improves the quality of life for people with severe and symptomatic tricuspid regurgitation. Post-implantation of the clip device, the patients can significantly eliminate symptoms such as shortness of breath, fatigue, decline in endurance, ascites, and peripheral edema.
The six-month pivotal TRILUMINATE CE Mark study examining edge-to-edge repair technique using Abbott's new clip therapy demonstrated a significant reduction in the severity of debilitating TR, sustained symptomatic improvements and enhanced functional status of the heart. Therefore, the physicians consider the device to be highly safe, durable, and reliable for the treatment of TR in high-risk patients or people with moderate or severe TR. The TriClip™ device has the potential to repair the tricuspid valve without open-heart surgery only by clipping together a portion of leaflets to reduce the blood flow. The minimally invasive approach allows the heart to pump blood more efficiently and relieve symptoms associated with the tricuspid valve leakage.
Although Abbott's TriClip™ device is built upon the same technology of the company's MitraClip transcatheter mitral valve therapy that is employed to treat leaky mitral valves, the next-gen device differs in terms of the delivery system. The new, steerable guiding catheter system is engineered to adapt the right side of the heart where the transcupid valve is present. To meet the needs of unique anatomies of patients, Abbott's TriClip™ is available in two different sizes (NT & XT). Currently, the TriClip™ is approved for use in Europe and other countries that recognize the CE mark.
The TriClip™ system consists of mainly two parts, steerable guide catheter (SGC) and clip delivery system (CDS). The CDS includes a steerable sleeve, delivery catheter, and wide chrome cobalt clip of 4 mm (NT size) and 7 mm (XT size) with articulated arms. The SGC in the new TriClip™ design contains two knobs for steering maneuvers. Besides, the steerable sleeve includes only one knob intended for deflecting the tip and reducing the radius of the distal curve. The modifications facilitate the bending and guiding of maneuvers into the right atrium. The device is used to clip the two leaflets together at the center and create two smaller openings to form a tighter seal. The upgraded TriClip™ allows clinicians to customize the procedure to each patient's unique valve and independently grasp leaflets before fastening. With 100% implant success rate, the device can help to save time and costs of patients by reducing the need for hospitalization by 40%.
Other Alternative for Minimally Invasive Tricuspid Therapy
The PASCAL repair system offers a minimally invasive approach for patients as the device is inserted through the groin area making an entry point of 0.7-0.8 cm. The independent clasping function of the PASCAL repair system provides some benefits to the physicians. During the surgery, the surgeon can individually control each side of the clasp machine, which could not be performed earlier. The technique allows the physicians to reopen one side of the clasp while keeping the other clasp in the stationary position, which helps to optimize its capture and reclose the system. The independent leaf capture method enhances the efficacy of the PASCAL system and provides optimal results. The patient can be easily extubated post-procedure and sent to normal ward. Unlike with valve surgery, the patient does not require pain medications and can be discharged within two days. After a week, the patient can even return to their daily activities and live a normal life.
The relatively new tricuspid therapy by Edward Lifesciences, known as FORMA, has been designed specifically for the treatment of leakage in tricuspid leaflets, but the medical device is currently under clinical trials and has yet to receive CE approval.
According to TechSci research report on "Global Interventional Cardiology Devices Market By Product Type (Angioplasty Balloons, Angioplasty Stents, Structural Heart Devices, Catheters, Plaque Modification Devices, Hemodynamic Flow Alteration Devices, Others) By End User (Hospitals & Clinics, Ambulatory Surgery Centers, Others) By Region, Competition Forecast & Opportunities, 2026", the global interventional cardiology devices market is anticipated to grow at a significant CAGR during the forecast period. The growth can be attributed to advancements in medical devices and rapid surge in the demand for minimally invasive surgeries. Additionally, high demand for novel products for the treatment of cardiovascular diseases to reduce risks and rising incidences of heart problems are contributing to the growth of global interventional cardiology devices market.
According to another TechSci research report on "United States Heart Valve Devices Market By Type (Mechanical Heart Valves, Biological Heart Valves, Transcatheter Heart Valves), By Product Type (Replacement (Aortic, Mitral, Others), Repair), By Valve Type (Tissue Valve, Mechanical Valve), By Procedure (Surgical, Transcatheter), By Application (Aortic Stenosis, Aortic Regurgitation, Mitral Stenosis, Mitral Regurgitation, Pulmonary Valvular Heart Disease), By Region, Forecast & Opportunities, 2025", United States heart valve devices market is expected to witness significant growth during the forecast period. The growth can be attributed to the increasing geriatric population and rising prevalence of cardiovascular diseases. Moreover, rapid advancements in healthcare infrastructure and easy access to healthcare services are boosting the growth of United States Heart Valve Devices market. In addition, rise in sedentary lifestyle and growing obese population are increasing the number of patients with heart problems, which is contributing to the growth of United States heart valve devices market.
No comments:
Post a Comment