The application of a real-time Polymerase chain reaction (PCR) system in the field of molecular diagnostics has become a standard method for identifying nucleic acids from collected microbial samples. The real-time PCR (also known as RT-PCR) systems are sensitive, rapid, precise, and minimize the risk of contamination while being employed for various research procedures such as detection and expression analysis of genes, quantitative genotyping, early diagnosis of diseases, forensics, etc. Ever since the introduction of RT-PCR, the technology is just getting better with each medical innovation. The COVID-19 pandemic has put RT-PCR in the spotlight as it is considered the most accurate and efficient testing method for the detection of the SARS-CoV-2 virus. The use of RT-PCRs has been tremendously expanding for the testing of novel coronavirus and the demand is likely to grow due to the rising incidences of infectious diseases.
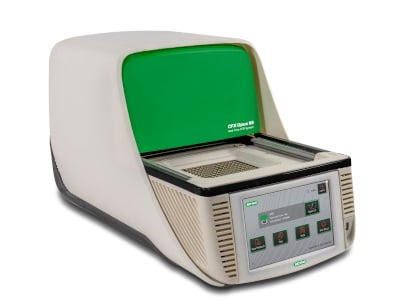
Recently, Bio-Rad Laboratories introduced the next generation of the company's CFX real-time PCR systems, CFX Opus 96 and CFX Opus 384, that offers improved connectivity and high performance. The sleek and modern CFX systems bring forth the next revolution in qPCR with their easy-to-use interface and advanced connectivity options. Both the RT-PCR systems are ideal for basic and translational research, genomic testing, quality control, process development, detecting infectious diseases, as well as pathogen detection. Along with expanding Bio-Rad's portfolio of CFX products, the company also launched cloud-based instrument connectivity and data management system, BR.io.
Consolidating an accurate optical shuttle system and improved thermal performance, the new CFX systems are designed to provide more consistent data than ever. The advanced RT-PCR systems are capable of performing stand-alone operations, which makes them indispensable as life-research tools for diagnostic interventions. The systems show excellent compatibility to ethernet as well as Wi-Fi, and thus enable completely wireless operations and data management. The user can transfer the retrieved data by connecting the CFX systems with a PC or via direct data transfer to a USB device. In network-connected locations, the CFX Opus system can be programmed to send the data as well notifications directly to your email or local drive, and thus the updated systems deliver an efficient experience to RT-PCR users.
Bio-Rad Laboratories' CFX Opus 96 has five color detection formats whereas CFX Opus 384 RT-PCR provides four color detection formats. The sensitive and modern touchscreen of the systems is responsive even with double gloves and more intuitive than previous generation CFX systems. However, the uniform optical system does not have regular calibration requirements, which saves time and effort. Additionally, the products' block design delivers tight thermal uniformity during the real-time PCR experiments.
The CXR Opus systems incorporate shuttle optics technology that utilizes the same excitation-detector pair just like other systems in the Bio-Rad's RT-PCR portfolio. In the new systems, shuttling the optics across the plate eliminates the possibility of variability that may result from detectors. Besides, the technology reduces the need for correction in detector angle arising from a single fixed camera. The consistency of the device is further augmented with the significant development in thermal performance, which provides uniformity of ±0.3â°C and accuracy of ±0.2â°C. Both the shuttle optics technology and the high-performance thermal gradient work brilliantly together and produce consistent and reliable results.
According to TechSci Research report on "Global dPCR and qPCR Products Market By Technology (Quantitative, Digital), By Product (Consumables & Reagents, Instruments, Software & Services), By Application (Clinical (Pathogen Testing, Blood Screening, Oncology Testing, Others), Research, Forensic, Others), By End User (Hospitals and Diagnostic Center, Research Laboratories and Academic Institute, Pharmaceutical and Biotechnology Companies, Clinical Research Organizations, Forensic Laboratories), By Region, Forecast & Opportunities, 2025", the global dPCR and qPCR market is estimated to register a significant CAGR owing to rising incidences of infectious diseases as well as increasing application of biomarker profiling for disease diagnostics. Moreover, introduction of technological advancements in PCR technologies and high costs of advanced PCR devices might hinder the growth of dPCR and qPCR market.
According to another TechSci Research report on "Global Real Time PCR Market By Product (Instruments, Reagents & Consumables, Software & Services), By Application (Clinical (Pathogen Testing, Blood Screening, Oncology Testing), Research, Forensic, Others), By End-user (Hospitals & Diagnostic Labs, Research Laboratories & Academic Institutes, Pharma-Biotech, Clinical Research Organizations (CRO), Forensic Laboratories, Others), By Region, Competition, Forecast & Opportunities, 2025", the global real-time PCR market is expected to grow significantly due to increasing advancements in PCR techniques and introduction of funds and grants by government and healthcare organizations across the globe.
No comments:
Post a Comment